Elements with their Symbol and Atomic Number in alphabetical order
The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that. Atomic Number: 56 Atomic Mass: 137.327 amu Melting Point: 725.0 °C (998.15 K, 1337.0 °F) Boiling Point: 1140.0 °C (1413.15 K, 2084.0 °F) Number of Protons/Electrons: 56 Number of Neutrons: 81 Classification: Alkaline Earth Crystal Structure: Cubic Density @ 293 K: 3.51 g/cm 3 Color: Silver Atomic Structure.
- Manganese (symbol: Mn; atomic number: 25) isn’t considered as a free element in its natural form since it’s often combined with iron in minerals. As a processed metal alloy, it has several important industrial uses including in stainless steel production.
- The Chemistry Division's Periodic Table describes the history, properties, resources, uses, isotopes, forms, costs, and other information for each element.
| Symbol | Element | Atomic Number | Symbol | Element | Atomic Number |
| Ac | Actinium | 89 | Md | Mendelevium | 101 |
| Al | Aluminum | 13 | Hg | Mercury | 80 |
| Am | Americium | 95 | Mo | Molybdenum | 42 |
| Sb | Antimony | 51 | Ns | Neilsborium | 107 |
| Ar | Argon | 18 | Nd | Neodymium | 60 |
| As | Arsenic | 33 | Ne | Neon | 10 |
| At | Astatine | 85 | Np | Neptunium | 93 |
| Ba | Barium | 56 | Nh | Nihonium | 113 |
| Bk | 97 | Ni | Nickel | 28 | |
| Be | 4 | Nb | Niobium | 41 | |
| Bi | 83 | N | Nitrogen | 7 | |
| Bh | 107 | No | Nobelium | 102 | |
| B | Boron | 5 | Og | Oganesson | 118 |
| Br | Bromine | 35 | Os | Osmium | 76 |
| Cd | Cadmium | 48 | O | Oxygen | 8 |
| Ca | Calcium | 20 | Pd | Palladium | 46 |
| Cf | Californium | 98 | P | Phosphorus | 15 |
| C | Carbon | 6 | Pt | Platinum | 78 |
| Ce | Cerium | 58 | Pu | Plutonium | 94 |
| Cs | Cesium | 55 | Po | Polonium | 84 |
| Cl | Chlorine | 17 | K | Potassium | 19 |
| Cr | Chromium | 24 | Pr | Praseodymium | 59 |
| Co | Cobalt | 27 | Pm | Promethium | 61 |
| Cn | Copernicium | 112 | Pa | Protactinium | 91 |
| Cu | Copper | 29 | Ra | Radium | 88 |
| Cm | Curium | 96 | Rn | Radon | 86 |
| Ds | Darmstadtium | 110 | Re | Rhenium | 75 |
| Db | Dubnium | 105 | Rh | Rhodium | 45 |
| Dy | Dysprosium | 66 | Rg | Roentgenium | 111 |
| Es | Einsteinium | 99 | Rb | Rubidium | 37 |
| Er | Erbium | 68 | Ru | Ruthenium | 44 |
| Eu | Europium | 63 | Rf | Rutherfordium | 104 |
| Fm | Fermium | 100 | Sm | Samarium | 62 |
| Fl | Flerovium | 114 | Sc | Scandium | 21 |
| F | Fluorine | 9 | Sg | Seaborgium | 106 |
| Fr | Francium | 87 | Se | Selenium | 34 |
| Gd | Gadolinium | 64 | Si | Silicon | 14 |
| Ga | Gallium | 31 | Ag | Silver | 47 |
| Ge | Germanium | 32 | Na | Sodium | 11 |
| Au | Gold | 79 | Sr | Strontium | 38 |
| Hf | Hafnium | 72 | S | Sulfur | 16 |
| Hs | Hassium | 108 | Ta | Tantalum | 73 |
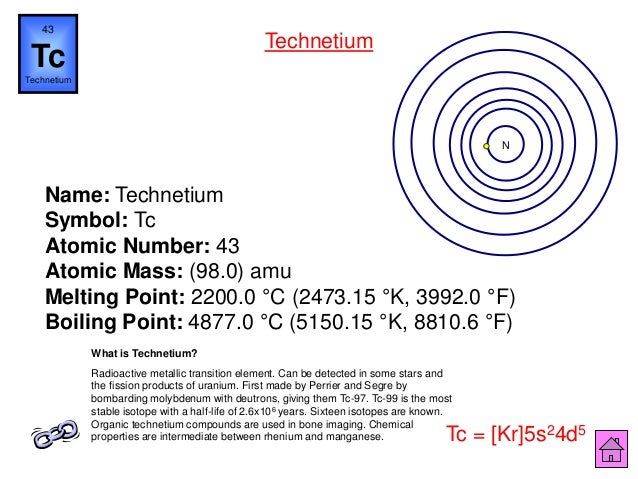
| He | Helium | 2 | Tc | Technetium | 43 |
| Ho | Holmium | 67 | Te | Tellurium | 52 |
| H | Hydrogen | 1 | Ts | Tennessine | 117 |
| In | Indium | 49 | Tb | Terbium | 65 |
| I | Iodine | 53 | Tl | Thallium | 81 |
| Ir | Iridium | 77 | Th | Thorium | 90 |
| Fe | Iron | 26 | Tm | Thulium | 69 |
| Kr | Krypton | 36 | Sn | Tin | 50 |
| La | Lanthanum | 57 | Ti | Titanium | 22 |
| Lr | Lawrencium | 103 | W | Tungsten | 74 |
| Pb | Lead | 82 | U | Uranium | 92 |
| Li | Lithium | 3 | V | Vanadium | 23 |
| Lv | Livermorium | 116 | Xe | Xenon | 54 |
| Lu | Lutetium | 71 | Yb | Ytterbium | 70 |
| Mg | Magnesium | 12 | Y | Yttrium | 39 |
| Mc | Moscovium | 115 | Zn | Zinc | 30 |
| Mn | Manganese | 25 | Zr | Zirconium | 40 |
| Mt | Meitnerium | 109 |
Manganese (symbol: Mn; atomic number: 25) isn’t considered as a free element in its natural form since it’s often combined with iron in minerals. As a processed metal alloy, it has several important industrial uses including in stainless steel production. It is, in fact, used to prevent rust and corrosion in steel via a process known as manganese phosphating, as well as being the cathode material in alkaline and zinc-carbon batteries.
Due to the important uses of manganese in modern society, the metal is considered as among the investments with a high return on investments. Like all metal-based investments, however, investors are well-advised to exercise relatively conservative investment strategies although aggressive strategies are also called for in certain instances.
Below is the historical Manganese price per metric ton.
| Year | Price | Price (Inflation Adjusted) | Change |
|---|---|---|---|
| 2005 | $1,515.00 | $1,967.82 | 0% |
| 2006 | $1,385.00 | $1,743.19 | -9% |
| 2007 | $3,210.00 | $3,930.12 | 57% |
| 2008 | $4,580.00 | $5,402.18 | 30% |
| 2009 | $2,390.00 | $2,830.36 | -92% |
| 2010 | $2,760.00 | $3,217.06 | 13% |
| 2011 | $3,480.00 | $3,930.52 | 21% |
| 2012 | $2,950.00 | $3,263.38 | -18% |
| 2013 | $2,330.00 | $2,539.42 | -27% |
| 2014 | $2,290.00 | $2,456.52 | -2% |
| 2015 | $2,140.00 | $2,293.32 | -7% |
| 2016 | $1,660.00 | $1,738.94 | -29% |
| 2017 | $1,850.00 | $1,894.40 | 10% |
| 2018 | $2,060.00 | $2,060.00 | 10% |

Brief History
Manganese has been used since ancient times although the etymology of its name is complex. In ancient times, the term “magnes” referred to two black minerals mined in Magnesia, now in modern-day Greece, said minerals of which were believed to have two distinct genders. The male magnes had magnetic properties and we now know it as magnetite or lodestone. The female magnes didn’t have a magnetic property but was instead used in decolorizing glass and, later on, became known as magnesia (i.e., manganese dioxide in modern times).
The modern name “manganese” was derived from the 16th century name for manganese dioxide, manganesum.
The pigments from several colored manganese oxides, such as manganese dioxide, have been used by men since the Stone Age, as is the case in the Gargas cave paintings. These have been dated as 24,000 to 30,000 years old.
The Egyptians and Romans also used manganese compounds in the manufacture of glass. These compounds were used to either remove or add color to glass, thus, the term “glassmakers’ soap” that are still in use today. Many of the best artisanal glassworks, especially the ones from 14th century Venice, have been treated with manganese compounds.
While manganese compounds was in widespread use among alchemists and glassmakers, it was only in 1774 when manganese as an element was first identified. Johan Gottlieb Gahn was the first one to isolate manganese via the reduction of dioxide with carbon.
The metal has since then been used in a wide range of industrial and commercial uses. In 1912, for example, patents were granted in the United States that allowed the use of manganese phosphate electrochemical conversion coatings as protection against corrosion and rust.
Price History
In 2015, the manganese industry has experienced a challenging year largely because of the economic downturn in China. According to certain publications, the metal has a price of $1.48/dry manganese ton – and it’s a decrease of 52 percent since the year’s start.
The low manganese prices have varying impact on the market participants although many are feeling the crunch. A few companies, such as Tshipi e Ntle and South32, are laying off permanent staff due to the challenging market conditions.

But investors are still optimistic as the manganese industry has experienced similar downturns in previous years yet recovered well enough to satisfy the desired returns on investment. The manganese prices will likely increase in the near future so everything should well on the front.
Use as an Investment
There is a wide range of companies that rely on the production and sales of manganese for their revenues. Most of these companies are listed on the stock exchange of several countries, such as the London Stock Exchange and the New York Stock Exchange, so stock investments are possible. Most of the mining companies also have diversified portfolios in the sense that, aside from manganese, other metals are also being mined.


What It’s Used For
Manganese is a highly valued metal because there are no satisfactory substitutes for it, particularly in its major metallurgical applications. But in its minor applications, such as in rust and corrosion protection, zinc is a good substitute.
The major applications for manganese include:
Isotope Of Manganese Atomic Number
- The production of steel and iron, thanks to its alloying, deoxidizing, and sulfur-fixing properties. The steelmaking industry accounts for 85 to 90 percent of the worldwide demand for manganese. The metal is used in stainless steel, in military helmets, and in Hadfield steel.
- The production of aluminum alloys, which accounts for the second-largest application of the metal. Aluminum alloy with manganese has increased corrosion resistance, thus, its widespread use for beverage cans.
- The production of unleaded gasoline as methylcyclopentadienyl manganese tricarbonyl. This is used in reducing engine knocking and increasing octane rating.
- The manufacture of chlorine and oxygen, as well as in drying black paints, in the form of manganese dioxide (MnO2).
Of course, manganese compounds are still being used as pigments in various applications, such as in adding color to glass and ceramics. Green- and pink-colored glasses likely have manganese compounds added.
Manganese Atomic Number Valence Electrons
The prices of manganese in a free market is then of special interest to several diverse industries from steel and glass production to battery manufacture. Be sure to monitor them so that you, too, can take advantage of the good returns on investments that the metal provides for its savvy investors.
